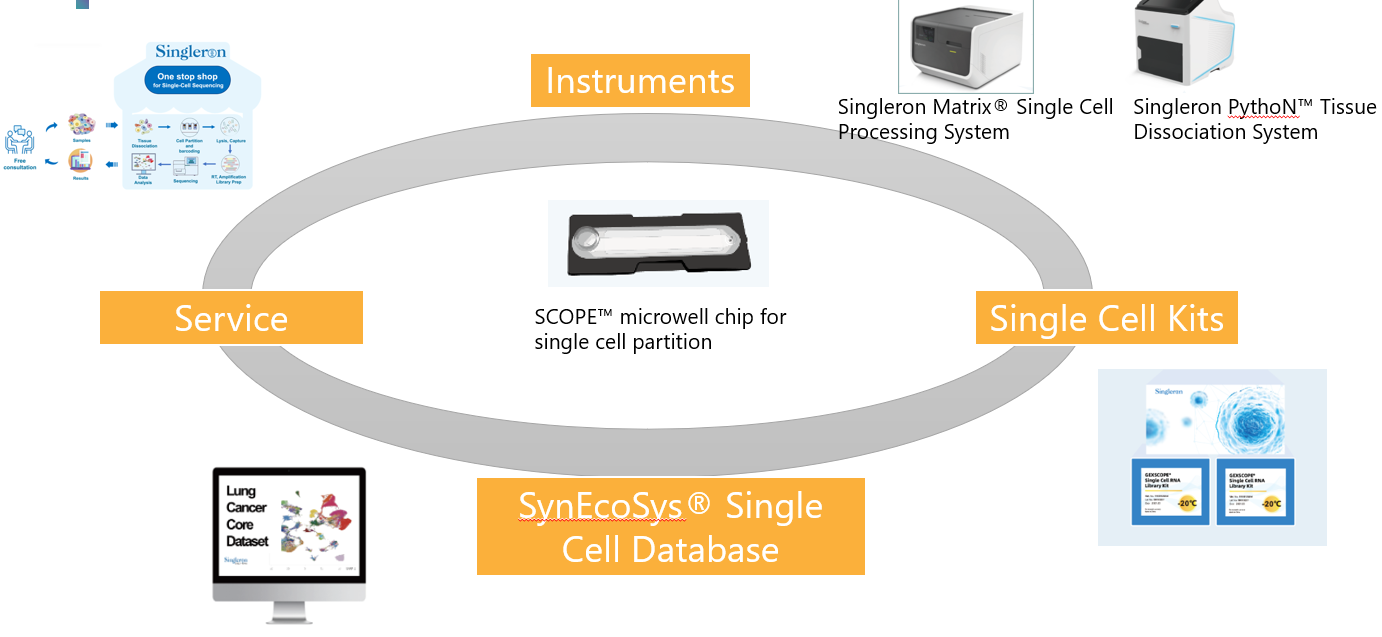

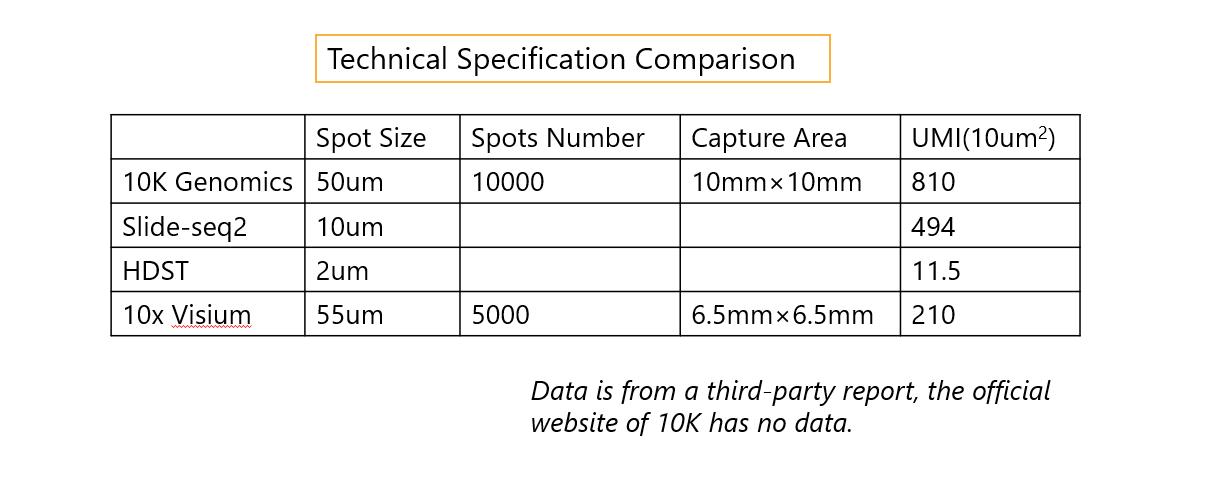
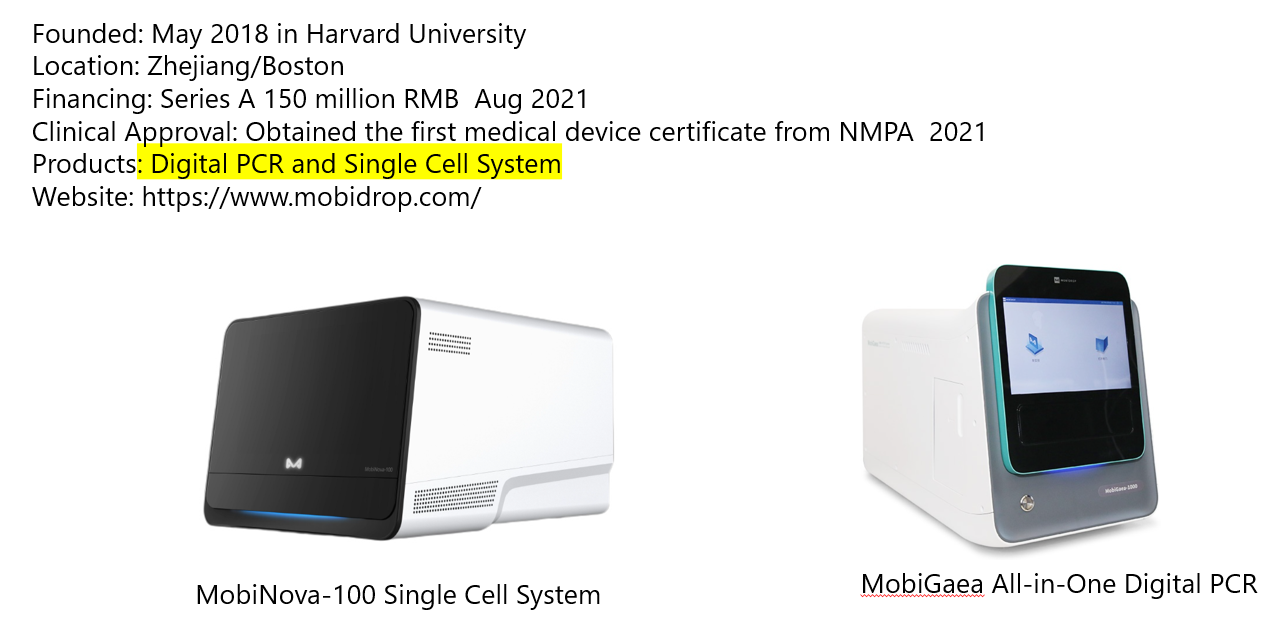


on going










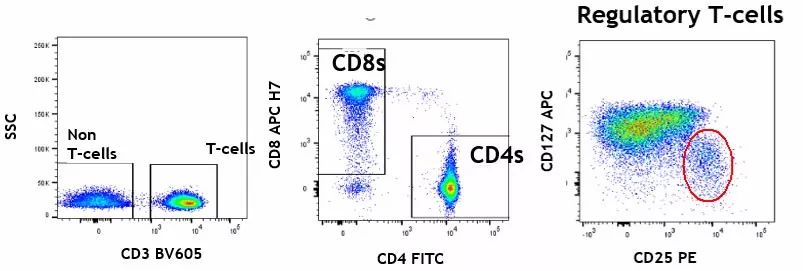
【实验结果】
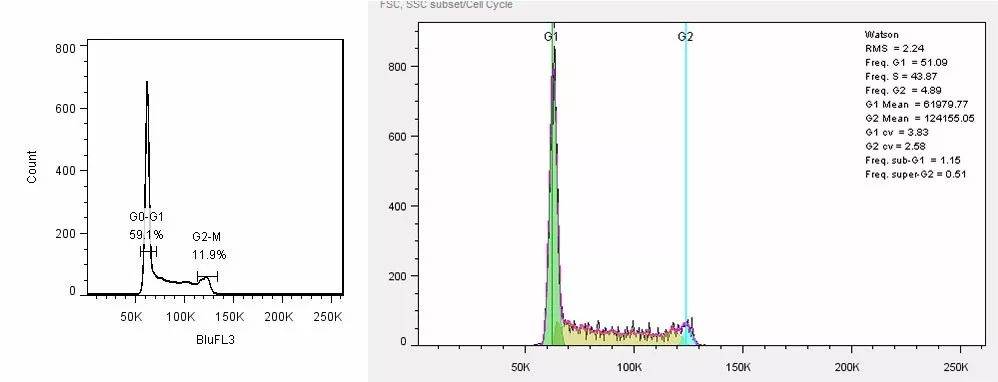
采用FlowJo软件自带的Cell Cycle工具进行分析,其中G1 mean:61979.77,G2 mean:124155.05,G2:G1=2.003。
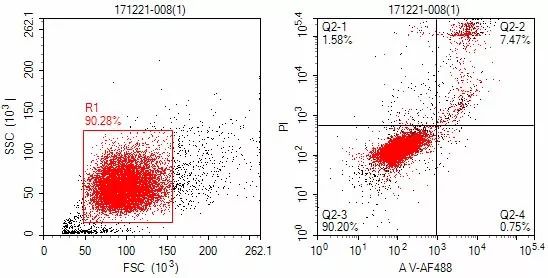
6. 加入适量TNF-α-FITC,INF-γ-PE,按照试剂盒说明进行孵育;
7. PBS洗涤,去上清,PBS重悬后上机检测。
【实验结果】
双阳性区域(Q2、Q6)为CD3+T细胞细胞因子分布。
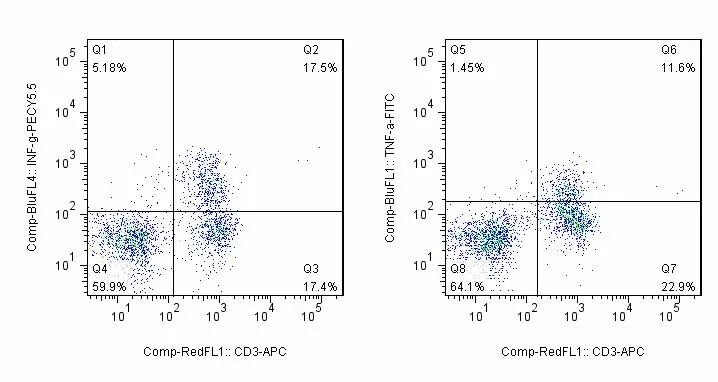
6. 洗涤:PBS洗涤2次并重悬,流式上机检测。
【实验结果】
下图Q1单阴性为TCRγδT细胞。
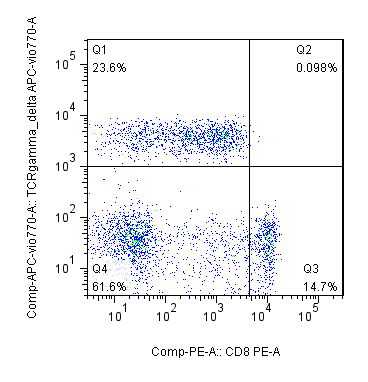
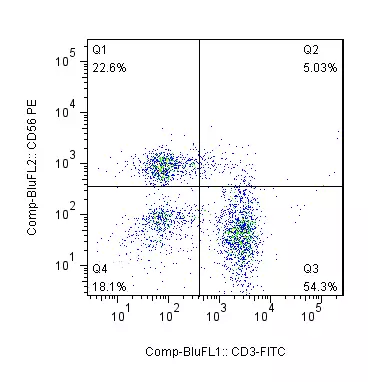
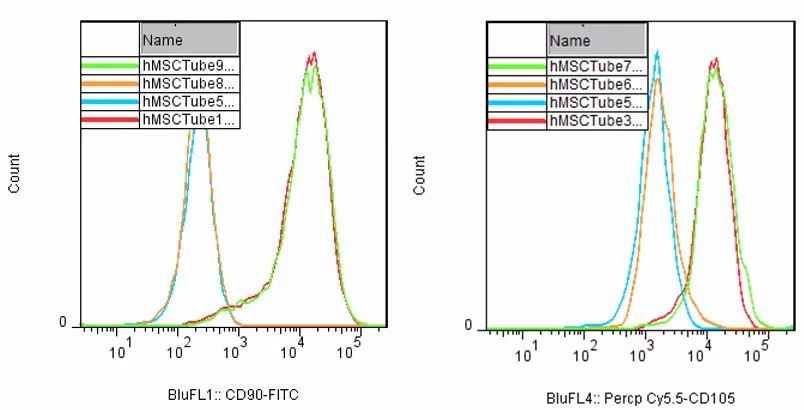
【实验结果】
Tube5为空白对照管、Tube1/3为单染管、Tube6/8为同型对照管、Tube7/9为多色样本管。显示,样本几乎不存在非特异性吸附,CD90和CD105阳性较强,其他未列出指标也都符合hMSC检测指标。
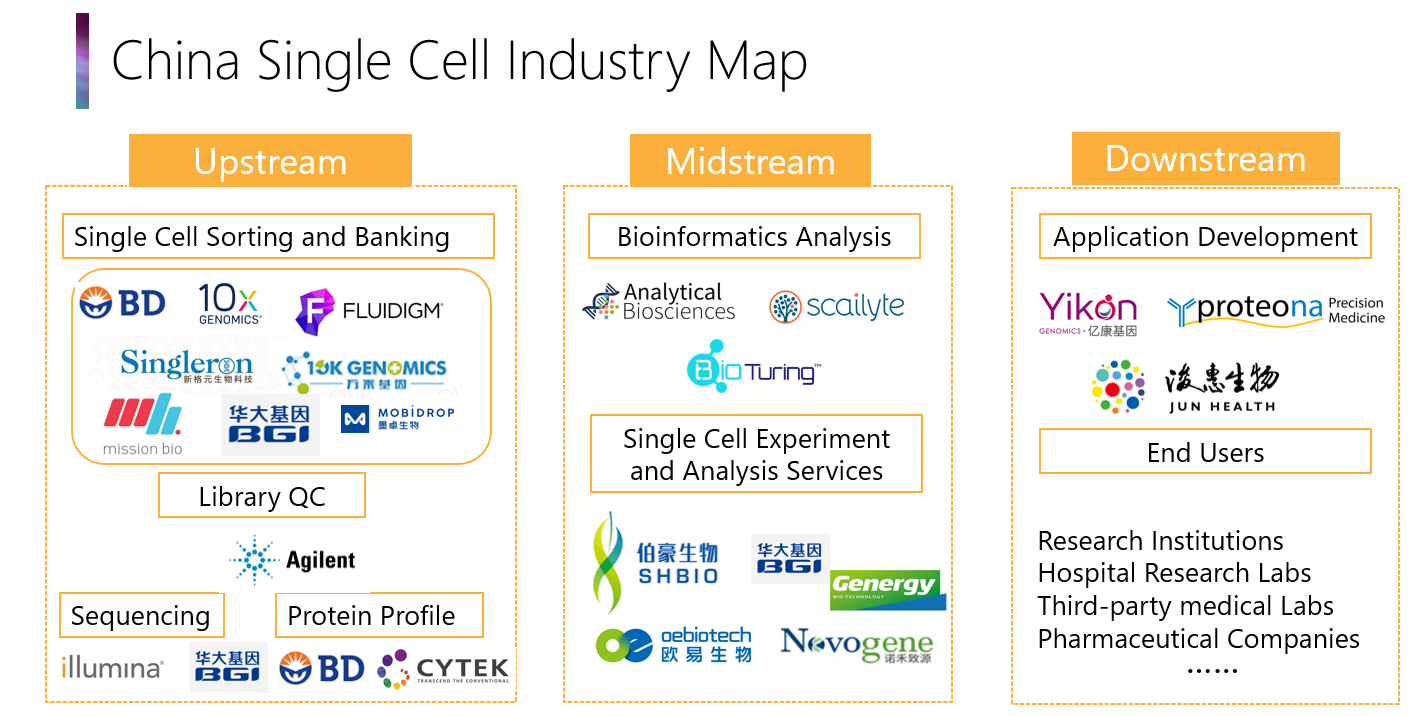
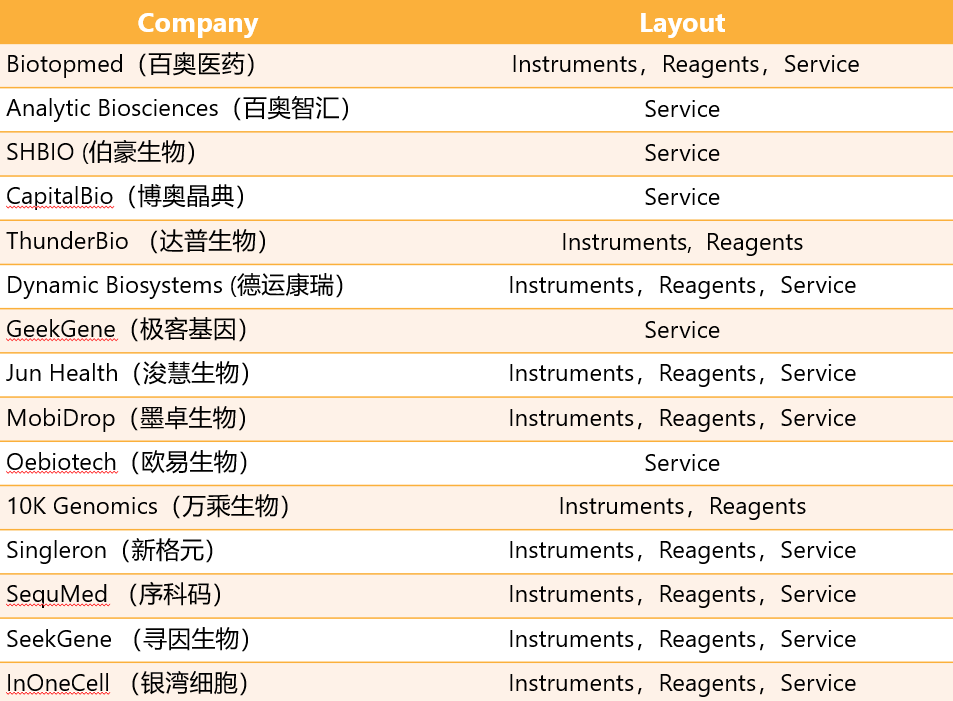
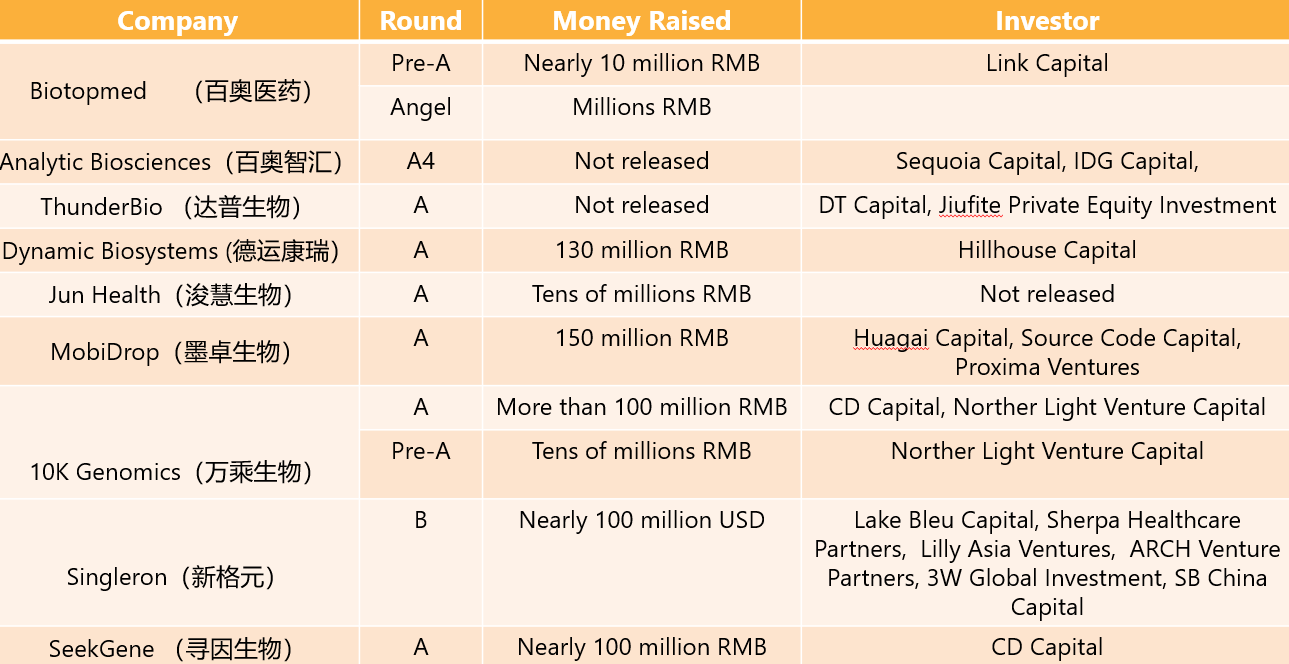
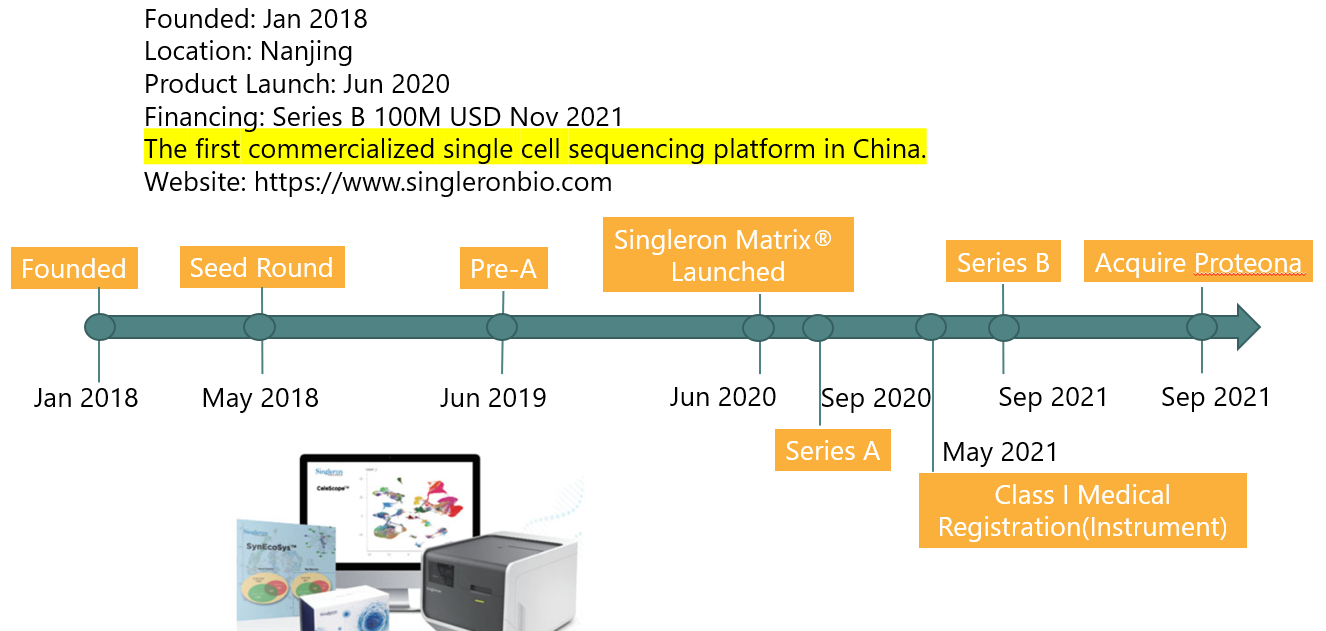
肿瘤不是一个简单的疾病过程,肿瘤免疫更是一个复杂的过程。恶性肿瘤病人往往免疫功能状态紊乱和低下,而免疫状态在一定程度上可预示着肿瘤的发展和预后。由于肿瘤免疫过程复杂特异蛋白少,或是肿瘤细胞或蛋白样物质掩盖了肿瘤抗原,使目前检查手段和蛋白分离方法尚不能检出肿瘤抗原。而通过实验性动物和对人类恶性肿瘤病人大量研究表明:免疫系统的所有有效应成分均对消除肿瘤细胞、控制肿瘤生长有作用,发挥免疫功能的淋巴细胞约占白细胞总量的20%,可分为T、B细胞、NK细胞、巨噬细胞和树突状细胞。通常采用单克隆抗体来分析细胞表面抗原分子,并对其分化群(簇,clusters of differentiation)进行了定义,用CD来描述白细胞表面抗原的不同成分。这样通过对恶性肿瘤病人相应免疫指标(CD分子)的检测,可对恶性肿瘤病人的预后进行判断并指导治疗和观察转归。
T淋巴细胞及表面抗原(CD4、CD8、CD3)与肿瘤的关系 大量研究工作已证实,宿主对机体内发生的肿瘤组织有自发性抵抗现象,而且以细胞免疫为主。众所周知,机体的细胞免疫由T淋巴细胞介导,T淋巴细胞的主要功能是调节蛋白质抗原引起的所有免疫应答,并清除细胞表面抗原或细胞内微生物的效应作用。T淋巴细胞进一步分化为辅助性T淋巴细胞(Th,CD4+/CD3+)和细胞毒性T淋巴细胞(Ts,CD8+/CD3+),对于抗原刺激的应答,辅助性T淋巴细胞分泌细胞因子。细胞因子可促进T淋巴细胞、B淋巴细胞、巨噬细胞的增殖和分化。McMichael认为T淋巴细胞作为细胞免疫调节的中心枢纽,Th和Ts细胞之间的平衡是通过CD4+和CD8+细胞之间的百分比表达出来的,其值下降表示免疫状态受抑制。目前研究还发现,CD4+细胞在协同杀伤肿瘤细胞中起着重要作用,CD4+细胞数减少可使肿瘤细胞发生免疫逃逸。亦有学者认为机体发生肿瘤时,在肿瘤局部的微环境发生免疫功能紊乱,表现为局部的CD8+亚群增高或降低,或识别肿瘤抗原上发生障碍,但恶性肿瘤病人的免疫功能直到很晚才发生。对于大多数肿瘤细胞来说,肿瘤细胞表达CD8+而不是CD4+,CD4+不能辨认肿瘤细胞,而是依赖于抗原提呈细胞,如果相关的肿瘤抗原被巨噬细胞提呈(DC),则对CD4特异性激活后才分泌淋巴因子激活CTL细胞、巨噬细胞和B细胞,产生其他淋巴因子和淋巴毒素和肿瘤坏死因子,肿瘤坏死因子可溶解肿瘤细胞。本文通过对50例恶性肿瘤病人和31例健康人进行外周血FCM检测,肿瘤组CD3、CD4+/CD3+、CD4/CD8比值明显低于对照组,肿瘤组CD8+/CD3+明显高于对照组,这表明恶性肿瘤病人的细胞免疫明显低下(P<0.05或P<0.01)。
B淋巴细胞及表面抗原(CD19、CD20)与肿瘤的关系 肿瘤的体液免疫是B细胞及抗体依赖的杀伤作用。B细胞表面免疫球蛋白与肿瘤抗原结合,处理和递呈肿瘤抗原,从而诱导T细胞对肿瘤的应答。B细胞所产生的抗体是多克隆异源性抗体。CD20是一非免疫球蛋白产物,参与细胞激活,是B细胞的特异性标志,前B细胞至活化B细胞时表达这一分子。而CD19作为全B细胞表面标志性抗原,是B细胞活化的共受体,在B细胞活化后消失,在外周血中正常分布为8%~15%。通过对CD19与CD20的检测,可在一定程度上反映出机体体液免疫功能状态。本文对50例肿瘤病人的CD19、CD20进行检测,其中恶性肿瘤病人组CD19与CD20明显低于健康对照组(P<0.01),表明肿瘤体液免疫也明显受抑制,与文献报道一致。
NK细胞及表面抗原(CD16、CD56)与肿瘤的关系 NK细胞是正常机体中对肿瘤细胞具有高度细胞毒性作用的淋巴样细胞,是一种广谱的杀伤细胞,对阻止肿瘤生长起重要作用。NK细胞是不同于T 、B淋巴细胞的淋巴细胞群,它们在体内相对较少,它们来源于骨髓的大颗粒细胞。它们不需预先致敏即能分泌细胞毒因子,从而杀伤肿瘤细胞。虽然NK细胞无靶细胞特异性,但在缺乏抗体和ADCC效应时,它们表现几种水平的靶细胞选择性:首先,它们对肿瘤细胞比对大部分正常细胞更具毒性作用;其次,不同的NK细胞克隆对不同来源的肿瘤类群表现不同的细胞毒模式。NK细胞代表了宿主抵抗原发和转移部位肿瘤生长的第一道防线,并通过T细胞补充特异性抗肿瘤应答。在某种意义上说,NK能强烈杀伤肿瘤细胞。有研究表明,体外介导杀伤大多肿瘤细胞的细胞亚群,90%以上是激活的NK细胞。NK细胞表面标志主要是CD16和CD56,其中CD16一般表达于未成熟NK细胞表面,CD56于成熟NK细胞表面,二者有交叉。其表达水平与NK细胞的整体活性具有相当的作用,其下降提示机体NK细胞作用受抑制,细胞免疫功能下降,不能有效发挥杀伤肿瘤细胞作用。陆云等认为CD16或CD56细胞数与NK细胞活性相关性随不同疾病及疾病不同阶段而变化。张峻梅等认为,肺癌病人NK细胞数与正常对照无显著性差异。本文对50例肿瘤病人和31例健康人的NK细胞即CD3+/CD16+56+进行比较,恶性肿瘤病人NK细胞较对照组显著升高(P<0.01)。这并不说明恶性肿瘤病人在细胞免疫和体液免疫降低时,NK细胞数量和活性增加。NK升高只是一种假象,因为测定时T+B+NK的值应在95%~105%之间,而T、B细胞值均降低,为了维持总淋巴细胞数量的恒定,NK的测定值升高,其实恶性肿瘤病人的NK细胞活性是降低的。
活化淋巴细胞及表面抗原(CD25、HLA-DR)与肿瘤的关系 静止T淋巴细胞在接受刺激后可发生增殖活化而形成效应细胞,表现为细胞因子的分泌及细胞因子受体和粘附分子在细胞表面表达。T淋巴细胞活化需T细胞受体与相应抗原结合为第一信号,同时又必须辅以第二信号即共刺激分子的结合,而T淋巴细胞的分裂增殖是以细胞因子与IL-ɑ受体(IL-2R)的结合为启动信号的,故可以通过检测T淋巴细胞的CD3+/HLA-DR+、CD3+/CD25+等活化抗原来监测T淋巴细胞活化状态。本文通过对50例恶性肿瘤病人和31例健康人CD3/HLA-DR和CD3/CD25进行测定,发现恶性肿瘤病人CD3+/HLA-DR+显著降低(P<0.01),表明活化T细胞减少,但其中并不说明没有产生活化T细胞,而是因总T细胞减少而导致其数值降低;CD3+/HLA-DR-升高,静止T细胞则相应增多(P<0.05);CD3-/HLA-DR-显著降低(P<0.01),表明活化B、NK细胞也减少;而CD3+/CD25+无显著变化(P>0.05);体外的活化T淋巴细胞试验证明,许多细胞因子可增强HLA-DR的表达,如IFN、IL-1、TNF-2等,从而增强机体的细胞免疫功能和抗肿瘤效应。本文对10例恶性肿瘤病人应用白介素-Ⅱ和高聚生治疗3个月后,所测活化T、B、NK淋巴细胞较治疗前显著升高(P<0.01),表明T、B、NK细胞开始活化,而CD3+/CD25+细胞无明显增加,可能与白介素-Ⅱ治疗病例较少有关。此外,本文检测结果还发现CD3+与CD3+/HLA-DR+呈正相关性(r=0.49,P<0.01)。
并观察治疗前后各分子的变化,其中CD4+/CD3+、CD3+、CD4/CD8比值、NK细胞(CD3+/CD16+56+)、活化T淋巴细胞(CD3+/HLA-DR+),活化B、NK细胞(CD3-/HLA-DR+)较对照组均显著性增高(P<0.01)。这表明经过免疫治疗后,体内肿瘤分泌的可溶性免疫抑制因子减少,机体通过T细胞的免疫应答,导致CD4+T细胞活性加强,调节激活了T、B、NK细胞的功能,从而改善了机体的细胞免疫功能
随着激光器配置的增加和新型染料的上市,流式分析已经从早期的单激光2色,2激光6色发展到现在3激光13色甚至更高。
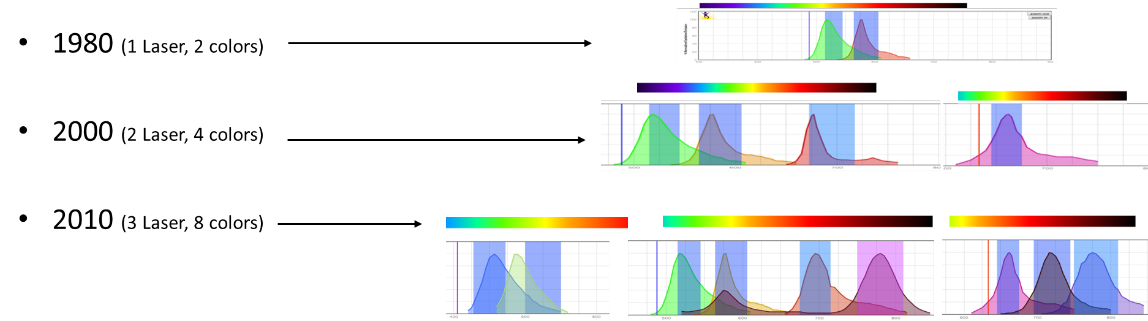
通过标记更多的荧光染料,我们可以检测细胞表面标志物或胞内分泌标志物探索细胞功能,并鉴定稀有细胞类型。然而,由于传统流式技术的限制,多色流式实验的开展还存在诸多困难:
1. 需要配置多激光(5激光)才能检测15色以上,成本高
2. 染料之间的补偿干扰大,实验设计要求高
3. 现有的染料可以满足多色实验搭配需求
4. 无法保证所有检测通道的高灵敏度
面对这一现状,来自Cytek公司最新研发的AuroraTM光谱流式细胞仪,通过其革命性的设计,使科学家不再受限于传统流式细胞技术,可以轻松开展多色流式实验。这一切是因为AuroraTM使用专利光波分复用技术,可以检测荧光染料从400nm到900nm的全部发射谱征;使用专利解析技术,即使发射光谱重合度很高的两个荧光染料,也可以轻松区别。同时借助全新设计的专利光路系统和电子信号处理系统,可以保证高灵敏度和分辨率。其主要特点如下:


与传统流式区别在于,不再是仅仅检测某一段区域的光谱发射波峰,而是检测并收集荧光被所有激光激发后在400-900nm内的全部发射谱征信息。这样就能够收集更多的信息(如同指纹一样),可以轻松区别在传统细胞仪上由于发射波峰高度重叠而无法同时使用的染料。(见下图)
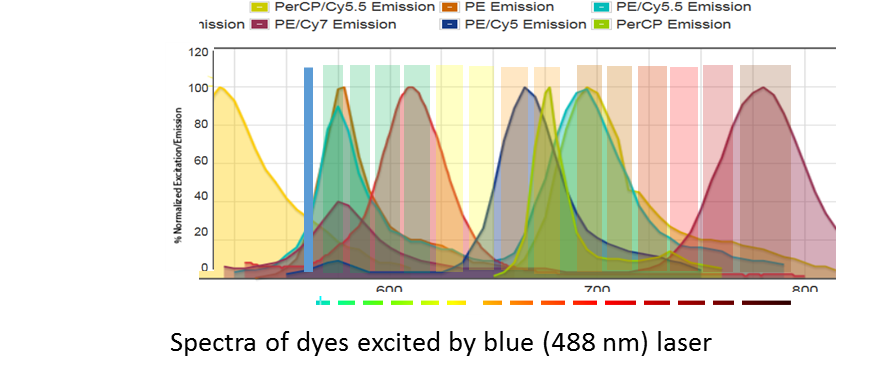 传统流式细胞仪的发射波峰检测方式(波峰重叠)
传统流式细胞仪的发射波峰检测方式(波峰重叠)
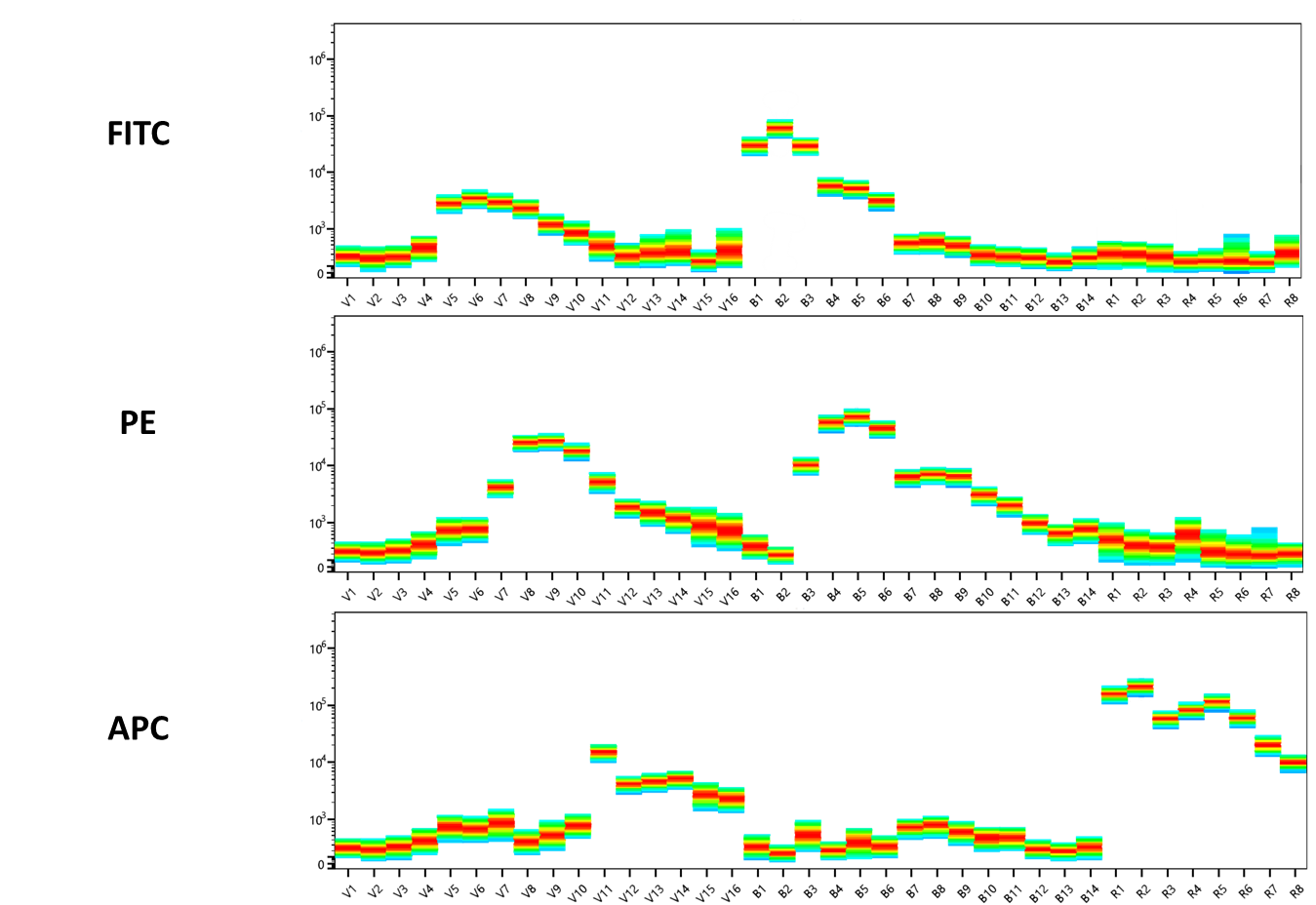 AuroraTM 光谱流式细胞仪检测染料的独特发射谱征(清晰区分)
AuroraTM 光谱流式细胞仪检测染料的独特发射谱征(清晰区分)
(横坐标为发射波长,纵坐标为荧光强度)
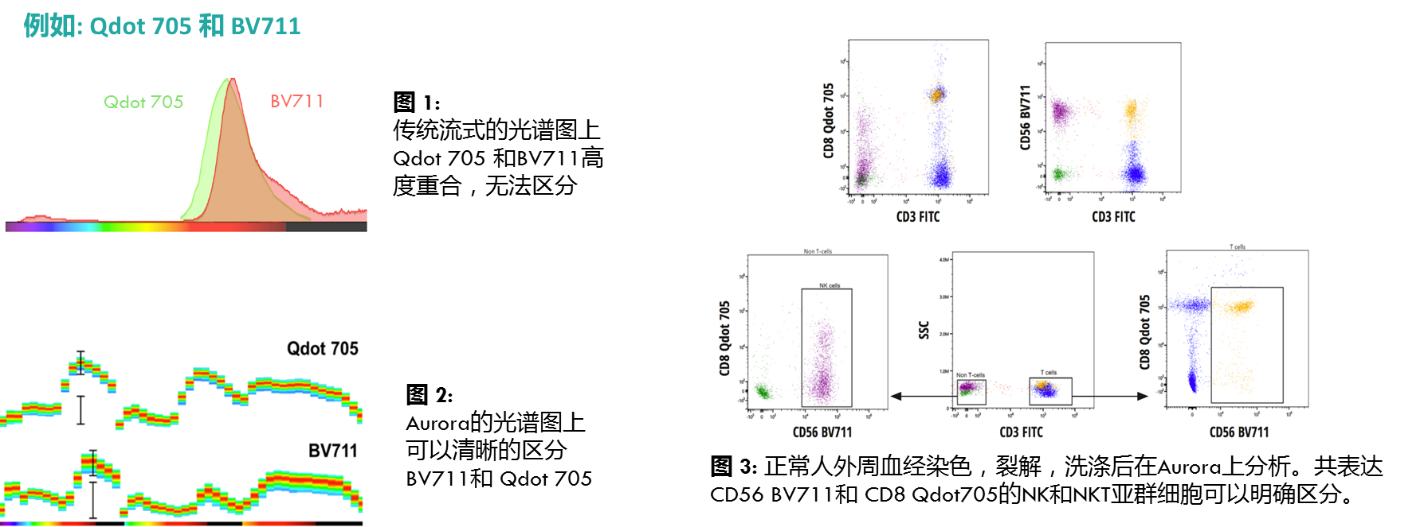
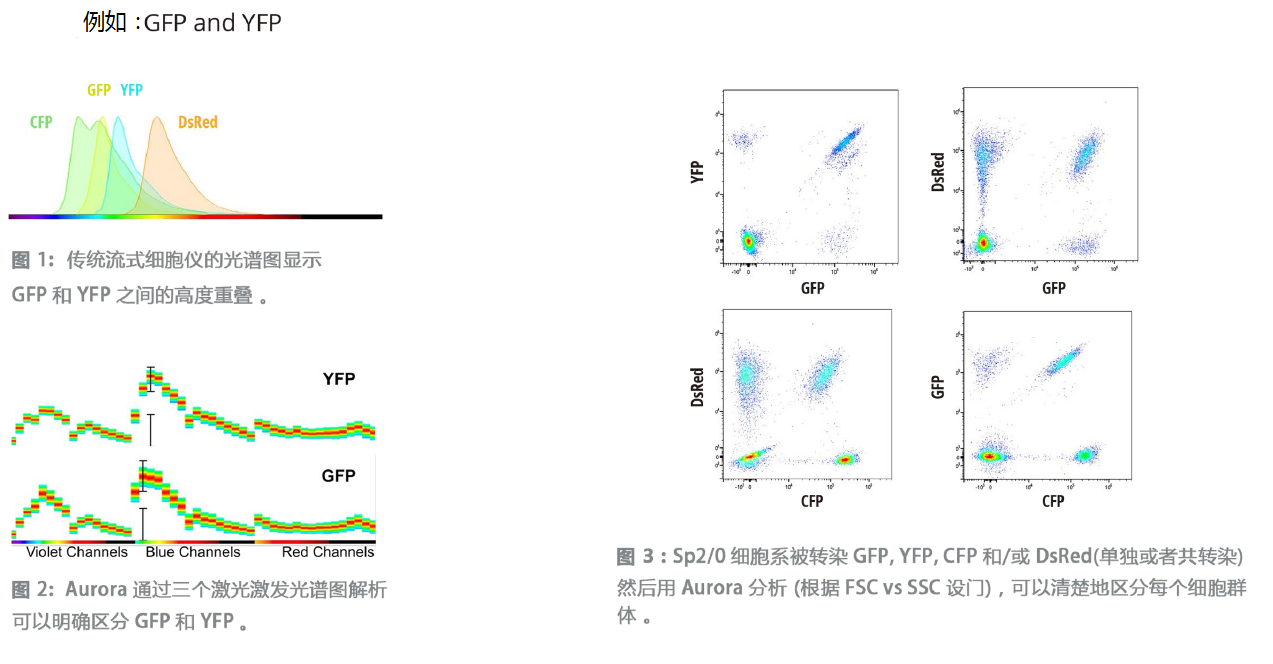
AuroraTM的这一创新技术带来的最大优势就是,我们在做多色实验选择荧光染料时更加灵活。原来在传统流式细胞仪上无法同时搭配使用的染料,现在都可以同时使用。
可以任意选择荧光染料,不受光谱重叠限制
下表为目前在AuroraTM 3激光系统上可以使用的染料列表
从上述的列表中选取20种染料搭配相关抗体,从下表可以看到,在传统流式细胞仪上,这些染料高度重叠,无法进行实验。
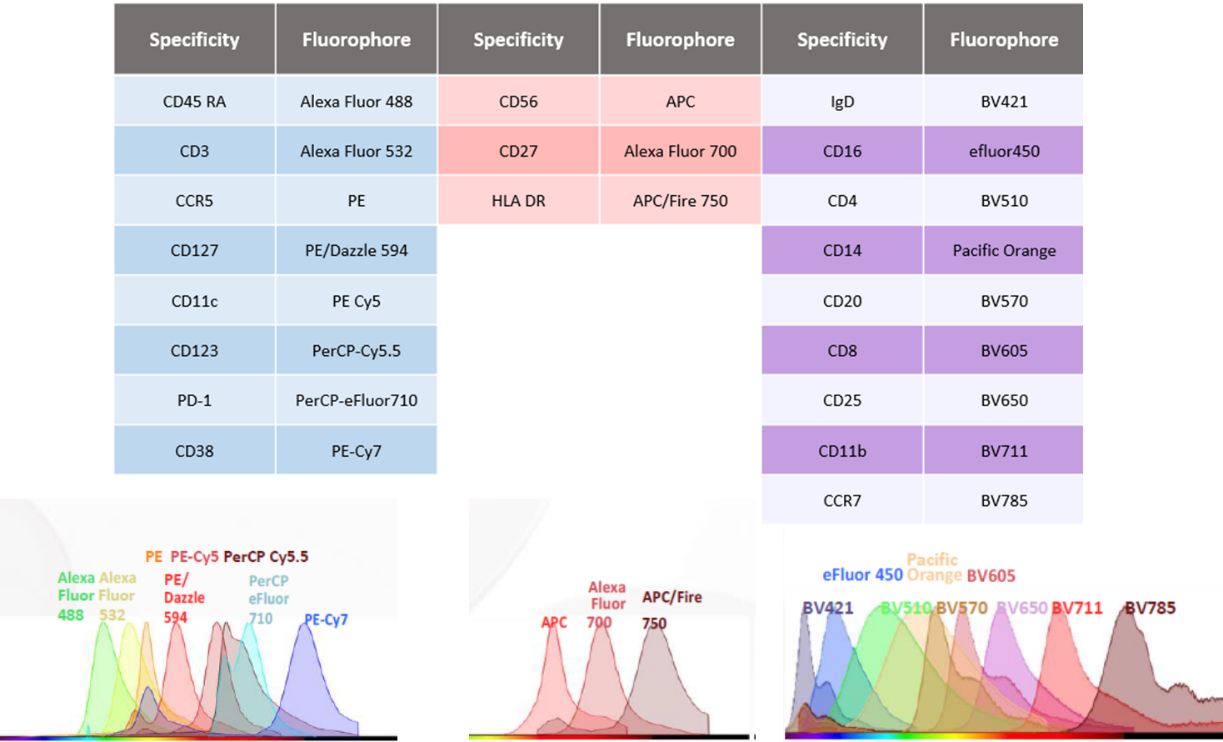
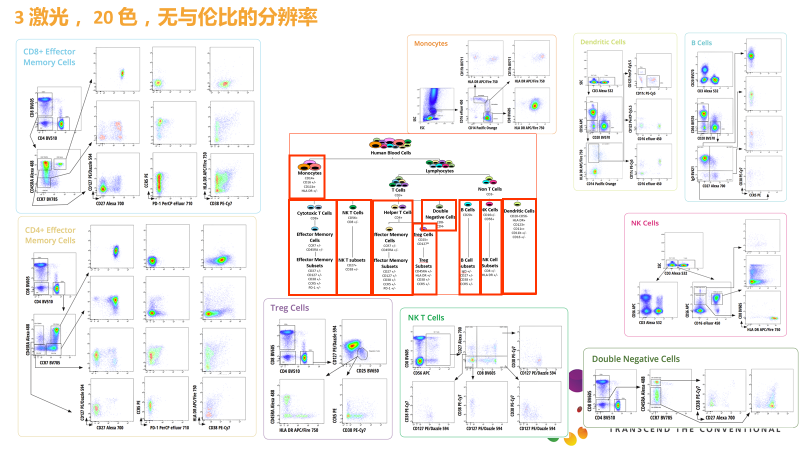 AuroraTM 20色组合实验数据
AuroraTM 20色组合实验数据
(详细信息请参考彩页)
三种不同技术比较表
| Cytek Aurora™ | Merck ImageStream | CyTOF | |
 |
 |
 |
|
| 产品概况 | Aurora使用全新的技术,可以检测荧光染料从400-900nm的全发射光谱,使用专利解析技术,即使发射光谱重合度很高的两个荧光染料也可以轻松区别。同时借助全新设计的专利光路系统和电子信号处理系统,可以保证高灵敏度和分辨率。 | 在传统流式荧光信号强度参数基础上,提供量化成像参数用于不同细胞群体的分析。 | CyTOF质谱流式细胞仪率先使用金属元素做为流式抗体、染料的标志物,利用质谱对标记细胞进行定量检测。 |
| 突出特点 | Aurora采用光谱专利解析技术,无需调节光谱之间的补偿,既继承了传统流式的特点又打破常规,实现3激光51通道的革命性跨越。 | ImageStream仪器设计重点偏向细胞成像,把流式的液流聚焦分离成单细胞技术和细胞荧光显微成像技术结合起来,通过细胞形态学观察,进行圈门。 | CyTOF通过与质谱技术相结合,采用金属元素标记物,再用流式细胞原理分离成单个细胞,获得单个细胞的原子质量谱,再进行数据转换实现细胞检测 |
| 细胞分析 | Aurora可沿用传统流式细胞分群圈门经验,各细胞群体分布情况比较固定,再结合其超高的灵敏度,即使是弱阳性信号也能进行精确区分。 | ImageStream可结合细胞形态综合参考进行圈门,但与传统流式圈门结果相差不大,可作为一个辅助和验证性工具。 | CyTOF采用金属元素标记,细胞亚群等群体分布与常规流式变化较大,不易确定目标细胞群。 |
| 操作便捷 | Aurora专用的SpectroFlo软件让你可以方便快捷的进行QC、实验设定、数据分析等,并带有荧光染料库,让你操作轻松上手 | ImageStream操作界面中,偏向于细胞形态展示,流式图像和数据的多样和灵活性欠缺,且数据量大,速度慢 | CyTOF把流式与质谱技术结合起来,复杂的数据转换,仪器成本较高,使用困难 |
| 检测通道 | Aurora单激光可以配置16个检测通道,5激光系统可以达到80个荧光检测通道 | ImageStream最多12个通道,>12色的实验就无法进行 | CyTOF虽检测通道数达到120个,但目前用来标记抗体的金属标签仅有30余种。 |
| 荧光染料选择 | 可以任意选择荧光染料搭配,灵活程度高,多色实验成本低 | 受传统流式技术限制,染料选择困难,根本无法满足多色流式需求。 | 只能使用特殊的金属标记抗体,成本昂贵,选择有限 |
| 性价比分析 | Aurora诠释了流式革命性进展,实验分析更精细,这是传统流式无法实现的,超高的性能和公平公正的价格实现超高性价比。 | ImageStream最高12个检测通道。辅助细胞成像技术,性能方面表现出后劲不足,价格比普通流式和荧光显微镜总价高出很多,性价比平平。 | 仪器硬件昂贵,后期使用耗材和试剂成本高,性价比低。 |
| 灵敏度 | 采用新的半导体检测器,光电转换效率是传统流式的10倍,而噪音则只有十分之一,具有超过灵敏度。 | 使用CCD作为检测器,灵敏度极差。 | 将细胞破碎之后进行分析,已经不是从完整的细胞功能角度研究,更类似于质谱检测方法,无法比较灵敏度。 |
DxP AthenaTM流式细胞仪系统使用DxP 技术,能够解决多色染色方案中弱表达群体难以检测的问题。多至3激光13荧光通道的配置供用户灵活选择,每种配置均能提供高灵敏度的分析功能。配备QbSureTM专利质控系统,确保实验结果的重复一致性。

可从2激光6色升级到3激光13色
提供每一个通道分辨率(Resolution)检测数值
高性能PMT和高效光路系统
FlowJo CE获取软件
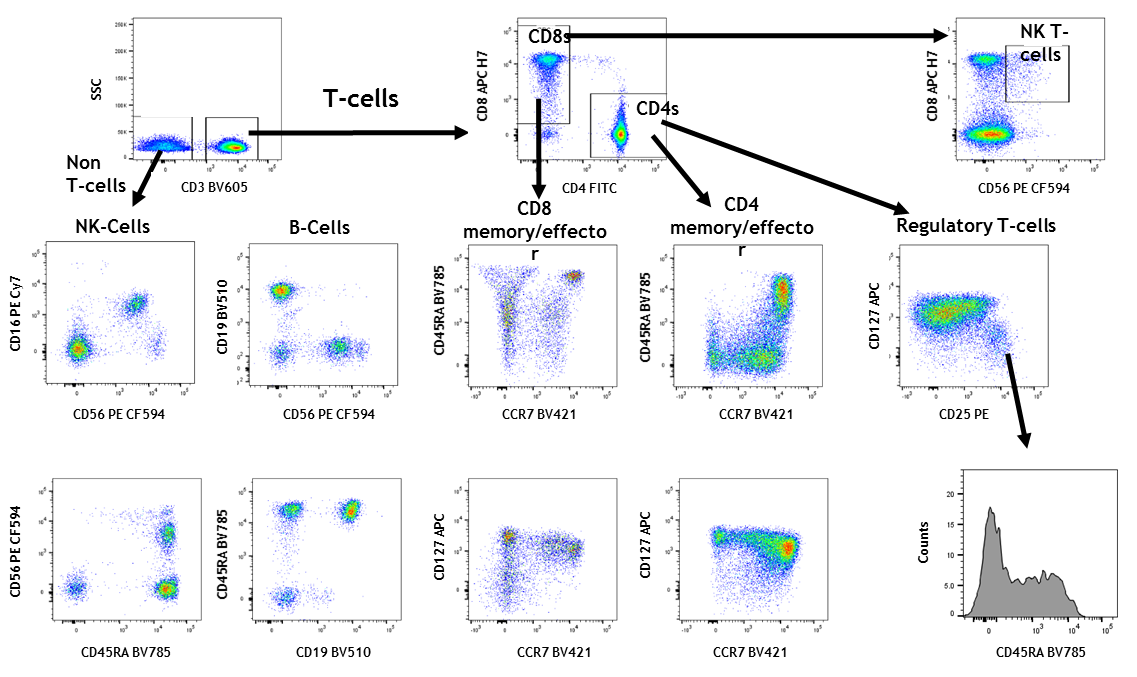
DxP AthenaTM 10色结果
Violet/Blue/Red 13-color
Blue/Red/Violet 10-color
Blue/Red/Violet 8-color
Blue/Red 6-color
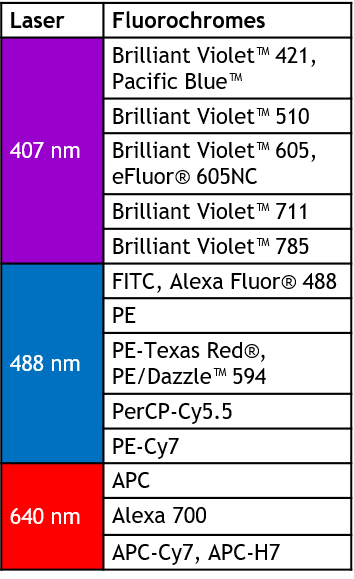
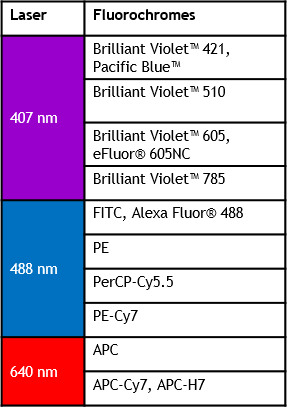
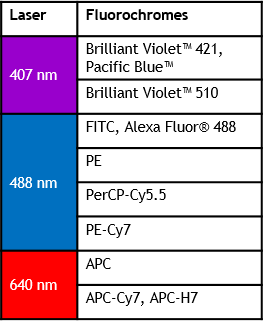
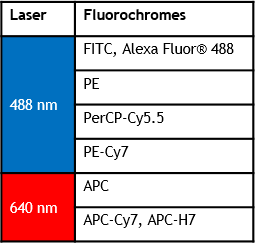

DxP AthenaTM 配置
Blue/Red/Violet 13-color